Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
JNC TN8400 2001-008, 36 Pages, 2001/03
Research on geologic disposal of high-level radioactive waste(HLW) has been underway in many countries. Bentonite exhibiting a low permeability, high swelling property and high sorption capacity for many radioelements is proposed as a buffer material in many countlies. Recently, cementitious materials are considered as candidate matelials for the geologic disposal of high-level radioactive waste. As the pH and the Ca, Na, K contents of hyperalkaline pore water from the cementitious materials are high, this hyperalkaline pore water would alter the buffer material. The main aim of this study is to investigate the effect of alkaline pore water into the bentonite. Used materials are montmorillonite, albite and quartz composing bentonite. These minerals mixed in a constant ratio (1:1wt%) made to react to distilled water and the alkali solutions (pH11-13). These studies have been conducted at temperatures of 50 - 150 C and run times of 10 - 200 day. XRD(X-ray powder diffraction) and SEM (Scanning Electron Microscopy) analyses were applied to studying the structure and quantitative data of each sample. From the result of this study, the main formed mineral of this experiment was analcime, which showed the tendency with a large amount of generation at a higher pH and temperature. Quantitative data of this study was conducted by X-ray powder diffraction method. THe order of the amount of the second analcime in each experiment is shown in the following. Montmorillonite and albite mixing test
C and run times of 10 - 200 day. XRD(X-ray powder diffraction) and SEM (Scanning Electron Microscopy) analyses were applied to studying the structure and quantitative data of each sample. From the result of this study, the main formed mineral of this experiment was analcime, which showed the tendency with a large amount of generation at a higher pH and temperature. Quantitative data of this study was conducted by X-ray powder diffraction method. THe order of the amount of the second analcime in each experiment is shown in the following. Montmorillonite and albite mixing test  Montmorillonite test
Montmorillonite test  Montmorillonite and quartz mixing test Activation energies (E
Montmorillonite and quartz mixing test Activation energies (E ) using the quantitative data of each test are shown in the following. (1)Montmorillonite test : 54.9kJ/mol (2)Montmorillonite and albite mixing test : 51.9kJ/mol (3)Montmorillonite and quartz mixing test : 59.6kJ/mol
) using the quantitative data of each test are shown in the following. (1)Montmorillonite test : 54.9kJ/mol (2)Montmorillonite and albite mixing test : 51.9kJ/mol (3)Montmorillonite and quartz mixing test : 59.6kJ/mol
*
PNC TJ1211 95-007, 117 Pages, 1995/02
1. Survey on leaching rate of pyrite, chlorite, epidote and siderite. (1) Pyrite Reaction rate (K) is depended on dissolve O concentration in Lin's literature. 1 - (1-x)
concentration in Lin's literature. 1 - (1-x) 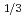 = k [O
= k [O ]
] 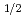 ・t (x: Mole number of dissolved FeS
・t (x: Mole number of dissolved FeS , [O
, [O ]: Dissolve O
]: Dissolve O concentration. T:Time) Reaction rate is changed by temperature. k = 2.2
concentration. T:Time) Reaction rate is changed by temperature. k = 2.2  10
10 exp(-9140/T) (2) chlorite Reaction rate is measured 2.7
exp(-9140/T) (2) chlorite Reaction rate is measured 2.7  6.7
6.7  10
10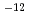 mol/m
mol/m /s(pH3
/s(pH3  4.5) by Swoboda-Colberg et al. Reaction rate is controlled by inner diffusion. (3) Epidote Reaction rate is measured 10
4.5) by Swoboda-Colberg et al. Reaction rate is controlled by inner diffusion. (3) Epidote Reaction rate is measured 10
 10
10 mol/cm
mol/cm /s in pH1
/s in pH1  11 by Rose. (4) Siderite Reaction rate is measured 9.93
11 by Rose. (4) Siderite Reaction rate is measured 9.93  10
10 mol/m
mol/m /s in O
/s in O -free solution by Greenberg et al. 2. Experimental study on leaching rate of minerals. (1) Measurement of leaching rate of minerals. Leaching rate of pyrite was measured in distilled waster under redox condition. Reaction rate (K) was estimated 10
-free solution by Greenberg et al. 2. Experimental study on leaching rate of minerals. (1) Measurement of leaching rate of minerals. Leaching rate of pyrite was measured in distilled waster under redox condition. Reaction rate (K) was estimated 10
 10
10 (cm
(cm ・mol
・mol ) 1/2・h
) 1/2・h by Lin's rate equation. At 56 days in the experiments. Alterated layer could not be found in SEM and EDX observation. (2) Measurement of leaching rate of chlorite Leaching rate of chlorite was measured in distilled waster under air condition. Disolution process of chloric was parabolic Stage, and reaction rate (K) was estimated order of 10
by Lin's rate equation. At 56 days in the experiments. Alterated layer could not be found in SEM and EDX observation. (2) Measurement of leaching rate of chlorite Leaching rate of chlorite was measured in distilled waster under air condition. Disolution process of chloric was parabolic Stage, and reaction rate (K) was estimated order of 10
 10
10 by Ross's rate equation.
by Ross's rate equation. 
 = kt [
= kt [  : disoleved mineral amount / intial mineral amount, t : time(sec) ] At 56 days in the experiments, alterated layer could not be found in SEM and EDX observation.
: disoleved mineral amount / intial mineral amount, t : time(sec) ] At 56 days in the experiments, alterated layer could not be found in SEM and EDX observation.
Ishikawajima-Harima Heavy Industries*
PNC TJ1150 94-001, 19 Pages, 1994/03
None
Sazarashi, Masami*; Ikeda, Yasuhisa*; Kumagai, Mikio*
PNC TJ1564 93-002, 23 Pages, 1993/02
None
*
PNC TJ1150 93-002, 253 Pages, 1993/02
no abstracts in English
; *; *; Tanimoto, Kenichi; Enokido, Yuji
PNC TN9410 92-208, 68 Pages, 1992/07
The plan of the gelogic disposal of high level waste that need to do estimate the effect of radiation in near field surrouding waste. We have applied "JOYO" spent fuel storage pool as irradiation field and investigated the effect of gamma radiation about quality of groundwater, because we have to obtained a basic data related geologic dispose under irradiaton condition. The experiment was applied artificial brine for simulated groundwater. The same samples were located in "JOYO" spent fuel storage pool without gamma radiation and other effects were estimated. The samples were also observed the variation of quality of artifical brine every fixed time after irradiation and were estimated the effect as the function of time, gamma irradiation were carried out from 24hours (1.0 10
10
 1.3
1.3 10
10 Gy) to 1440 hours (4.4
Gy) to 1440 hours (4.4 10
10
 6.8
6.8 10
10 Gy). The results indicate the following. (1)The change of pH, conductivity and ion concentrations in artifical brine could not be observed in the samples before and after irradiation. (2)Eh of the samples was 241mV before irradiation, but it decreased 156mV after irradiaton for 1440 hours. Eh tend to decrease by increase of the absorption dose. (3)Do of the samples before and after irradiation for 1440 hours were 20.76 and 5930
Gy). The results indicate the following. (1)The change of pH, conductivity and ion concentrations in artifical brine could not be observed in the samples before and after irradiation. (2)Eh of the samples was 241mV before irradiation, but it decreased 156mV after irradiaton for 1440 hours. Eh tend to decrease by increase of the absorption dose. (3)Do of the samples before and after irradiation for 1440 hours were 20.76 and 5930  g/
g/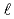 , respectively. Do tend to increases by increase of the absorption dose. (4)Before and after irradiation test for 480 hours, nitric ion was detected 2.9 and 105ppm, respectively, In no gamma irradiaton test, nitric ion was detdcted 4.0 and 5.6ppm, respectively. For 1440 hours, nitric ion was detected 15ppm after irradiation and 11ppm after rest without gamma irradiation. (5)pH, Eh, Do, conductivity and all ion concentrations in artifical brine have no the variation as the function ot time within fixed time (about 4hours) after irradiation. These results suggest that oxygen which were generated by the gamma radiolysis of water was incrased Do, ...
, respectively. Do tend to increases by increase of the absorption dose. (4)Before and after irradiation test for 480 hours, nitric ion was detected 2.9 and 105ppm, respectively, In no gamma irradiaton test, nitric ion was detdcted 4.0 and 5.6ppm, respectively. For 1440 hours, nitric ion was detected 15ppm after irradiation and 11ppm after rest without gamma irradiation. (5)pH, Eh, Do, conductivity and all ion concentrations in artifical brine have no the variation as the function ot time within fixed time (about 4hours) after irradiation. These results suggest that oxygen which were generated by the gamma radiolysis of water was incrased Do, ...